Project-
Blood & Plasma Re-labelling
The current Blood & Plasma relabelling process centres are solely reliant on using and topping throughout the day large amounts of hazardous & expensive dry ice to maintain the integrity of samples whilst they are being handled & worked on.
This cold chain process poses an increased potential for risk & failure, if they continue to be used.
Current Issues in Blood & Plasma Relabelling
Blood, plasma and platelets are required to be stored and transported at specific temperature ranges to maintain their integrity. After these samples leave their controlled freezer storage for relabelling, a huge emphasis is placed on the availability and long-term viability of dry ice to enable a safe environment. Given the importance of the goods and materials at stake—this could be a costly and out-dated strategy.
Relabelling & Safety
Cold chain requirements are posing costly and hazardous challenges to the current blood & plasma relabelling strategy. There is a real potential for injury and product loss, as the safe storage of these goods whilst being worked on is solely reliant on dry ice being used throughout the entire process.
The dry ice method currently being used means that staff are often required to “top-up” the tubs with dry ice during every standard working day and as we know, dry ice is considered a dangerous good itself. Issues relating to carbon dioxide release during sublimation have the potential to cause harmful effects on those exposed to the gases, explosions can occur if pressurised, and the ultra-low temperatures can cause skin-irritation and burns when handled incorrectly.
Dry Ice Cooling
Often, especially in regional areas, there is insufficient specialised medical freezers that are designed to store the sensitive material at the required temperatures. These locations will either be solely reliant on dry ice availability or will be forced to purchase further additional medical equipment for safe storage. Our 2n1 Freezer/ Workstations known “Clean-Air”, with holding temperatures of -40°C, can not only be used during the relabelling process but can also be used as a safe and reliable alternative for short-term storage solutions to sensitive goods in the laboratory.
Storage
Often, especially in regional areas, there is insufficient specialised medical freezers that are designed to store the sensitive material at the required temperatures. These locations will either be solely reliant on dry ice availability or will be forced to purchase further additional medical equipment for safe storage. Our 2n1 Freezer/ Workstations known “Clean-Air”, with holding temperatures of -40°C, can not only be used during the relabelling process but can also be used as a safe and reliable alternative for short-term storage solutions to sensitive goods in the laboratory.
SOLVING THE PROBLEM-
The challenges of today have allowed for a new way of thinking and for a new technological advancement—with regards to how we confront cold chain management, without the need for or the reliance on outdated cooling methods such as dry ice.
The innovative air-stream lid is an advancement that will instil confidence and trust in reliability, as contents and matter that are temperature sensitive can now be stored easily and safely, knowing that the integrity of the blood plasma will not be compromised. Whilst allowing for a more efficient handling process and by keeping staff safety at the forefront, the Clean-Air freezer solves the current cold chain management reliance and reliability issues. There is no better time than now, to employ this innovative and forward-thinking solution to revolutionise the relabelling process.
Designed locally before initial manufacturing stages are completed offshore, the Clean-Air freezer returns to Australia for the final assembly stages and rigorous quality checks to the system prior to distribution.

FEATURES & BENEFITS
Flexibility
The Clean-Air freezers offer efficient, reliable, low temperature cooling down to -40°C meaning that, in addition to being used during the plasma relabelling process, they can be used as a temporary storage unit in destinations that do not have appropriate or sufficient medical equipment. The open-air technology allows for easy to reach access and handling of goods being kept cool within the tub regardless of shape or handling requirements.

Low Maintenance
Clean-Air freezers require minimal ongoing maintenance to continue operating effectively. They have been designed for durability and repeated use over many years and the daily operating procedures required for plasma relabelling means there is no need for intensive maintenance schedules.
Safety
The open-air cooling system has enabled us to remove hazardous dry ice from the plasma relabelling process meaning staff will no longer have to handle dry ice or be concerned with potentially breathing in CO2 gases whilst working on the samples. All freezers are pre-programmed prior to delivery based on clients specific needs allowing for one-touch operation and reducing workplace health and safety risk through starting/finishing procedures.
Customisation
Freezers are fully customisable depending on your operational requirements. Temperature parameters and programming, LED display, internal chamber area, external dimensions and even benchtops can be customised to suit your needs. All freezers can be height adjusted so that staff are working in a safe environment.
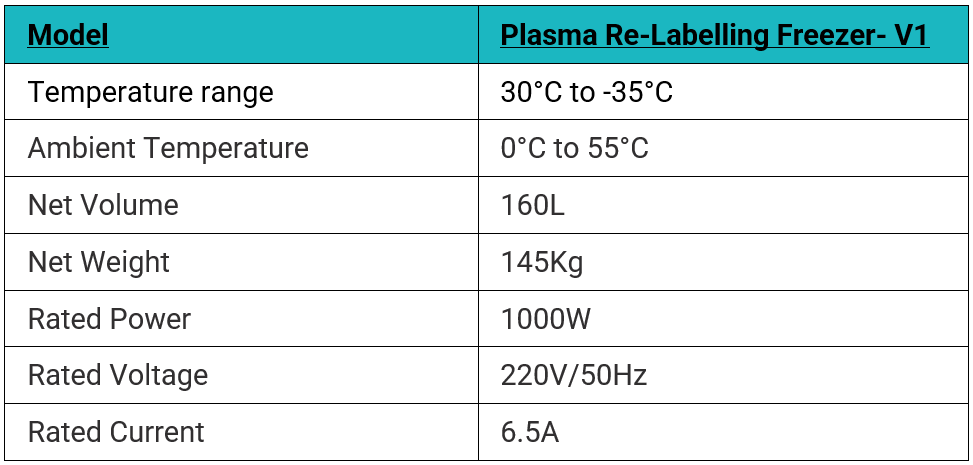
SPECIFICATIONS